What was once supposed to be a short and temporary lockdown has now stretched on for eight long months. When will it all end?
Recently, the news has announced that a vaccine is almost ready to be dispensed. It usually takes years of extensive research and testing before a vaccine can be safely available for use. However, due to the severity of the Covid-19 virus, a cure is needed faster than ever.
First starting in March, studies included deciphering the SARS- CoV-2 genome, and went through three stages of testing and verifying. The main goal was to stimulate the immune system and produce antibodies, which help to combat the disease. So, what are the different phases of producing a vaccine?
When a new virus is discovered, scientists go through the process of breaking it down and analyzing it. To produce an effective vaccine, they need to fully understand what it is and the effects it has. Once they begin to create possible vaccines, they test it on various cells and give it to lab animals. These often include mice or monkeys because their immune systems are most similar to humans. This stage is best known as the preclinical testing stage, where all possible vaccines are tested. For the Coronavirus, at the end of this stage, there were 87 possible vaccines in development.
Phase 1 determines whether or not the vaccine is safe to be administered via tests. This is to gather information on the efficacy of the vaccine, and how it may help the immune system.
Phase 2 involves expanded trials, where researchers administer the vaccine to hundreds of people. In other terms, each age group is separated, and closely monitored to observe how it affects people of different ages. This step helps to finalize the vaccine’s safety and ability to stimulate the immune system.
Finally, Phase 3 is when scientists give the vaccine to thousands of people, and are able to create a sort of mock population, where it closely resembles the actual human population, and they can see how easily the virus spreads afterwards. Additionally, based on the number of people in Phase 3, it can then become evident if certain side effects arise, because the genetic variation is both greater, and easier to monitor.
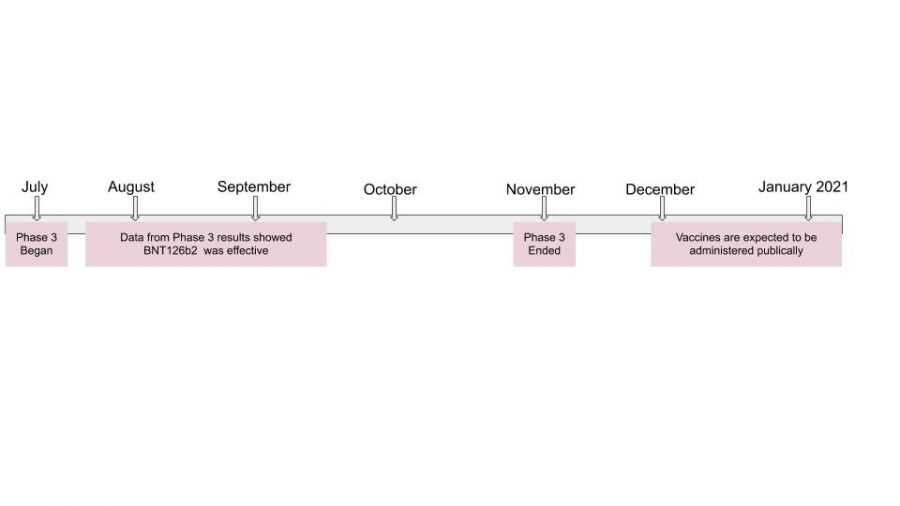
In January, vaccines were developed based on messenger RNA, or mRNA, which helps to produce certain proteins in the body. The pharmaceutical company Moderna developed this before most of the other companies, giving them a head start on development. The vaccine would then have to have specified information that when it would be injected into the body, it would make a certain protein that targeted Covid-19, by enacting the immune response. It then went through the filtering process and passed throughout the stages, where the potential BNT162 passed through all tests. Then, on July 27th, the Phase 3 clinical trial began, and tested on 43,538 participants, and 38,955 who received a second dose during November, showing its effectiveness.
Recently, companies have come a long way from what they were in March. The drug making company, Pfizer, was then able to show data in early November that their Phase 3 was proving to be 90% successful, says BioPharma Drive. They were able to conclude that their vaccination would prove protective against the ongoing virus. As of now, Pfizer needs to get approval from the Food and Drug Administration (FDA), for an emergency use drug, showing that the vaccine is almost ready, according to the New York Times. Moderna also plans to follow in the same footsteps in several weeks in hopes to gain approval.
The results of Phase 3 for many vaccine making companies showed that a mode to control and prevent Covid-19 could be developed. Both Pfizer and BioNTech are in the midst of analyzing the data from the second dosage which test candidates received. This is required for the FDA’s Emergency Use Authorization, especially since candidates are usually to be monitored for two years before releasing a vaccine, says Pfizer. By generating the correct and wanted data, the safety will be demonstrated, and the FDA can mass produce the product. If all goes well, the vaccines are expected to begin being administered to the people anywhere from December to January.